General
The ACSR inventory includes various types and numbers of biospecimens donated by individuals living with and without HIV who are not part of the ACSR’s epidemiological and clinical studies collections. Included are samples collected pre- and post- HAART from patients diagnosed with AIDS-defining cancers, non-AIDS defining cancers, opportunistic infections and reactive processes.
Domestic
ACSR Domestic Collection
Women’s Interagency HIV Study (WIHS) donations
San Francisco Young Men’s Health Study (SFYMHS)
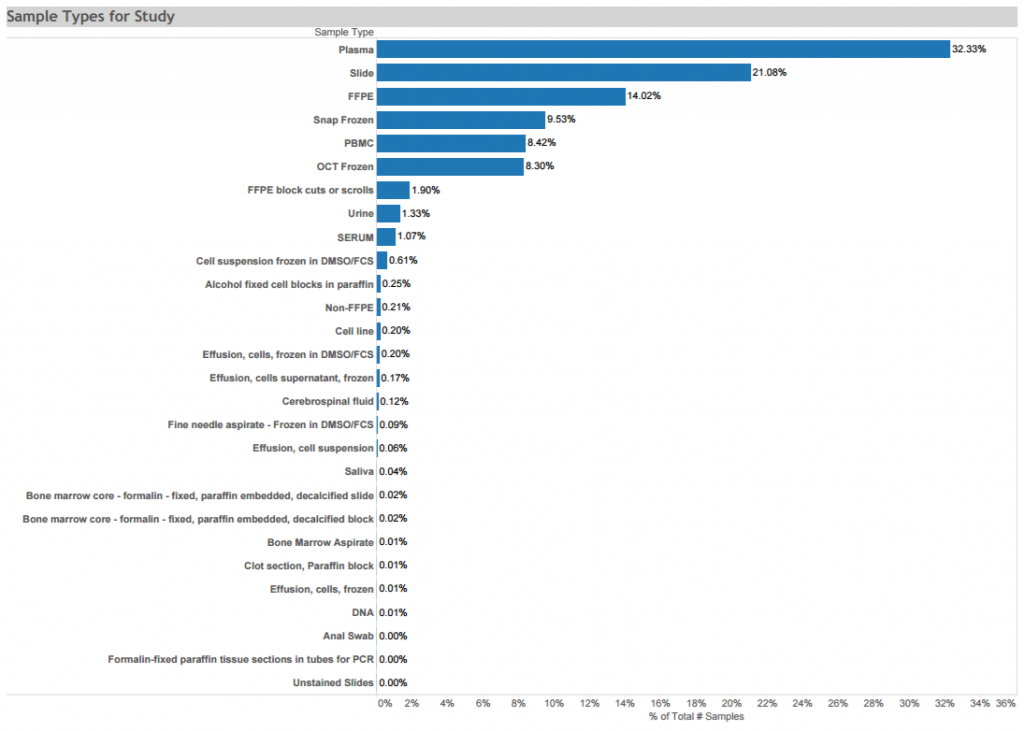
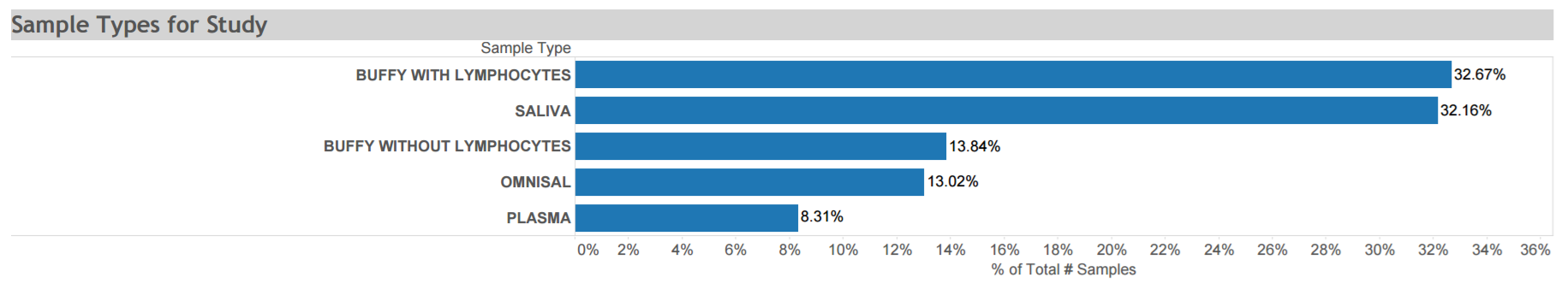
Women’s Interagency HIV Study (WIHS) donations: A longitudinal cohort study of HIV+ and HIV- adult women to characterize the long-term, natural and treated history of HIV infection and to provide insight into the changing demographics of the HIV epidemic in the United States. Data and biospecimen donations to the ACSR from 1400 women include plasma, PBMCs, saliva and a small number of cervical tissue samples that were collected at multiple time points from 1997 to 2015. Available biospecimens are depicted in the graphic. Click here for STUDY DETAIL
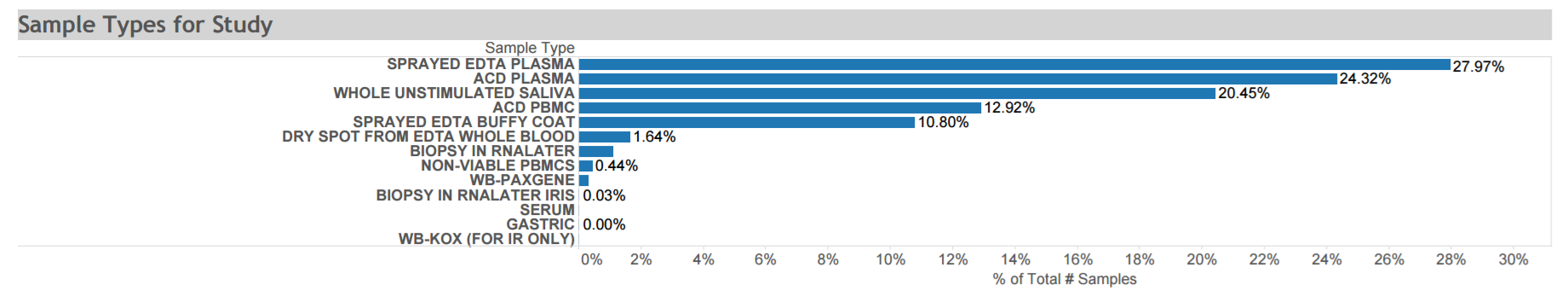
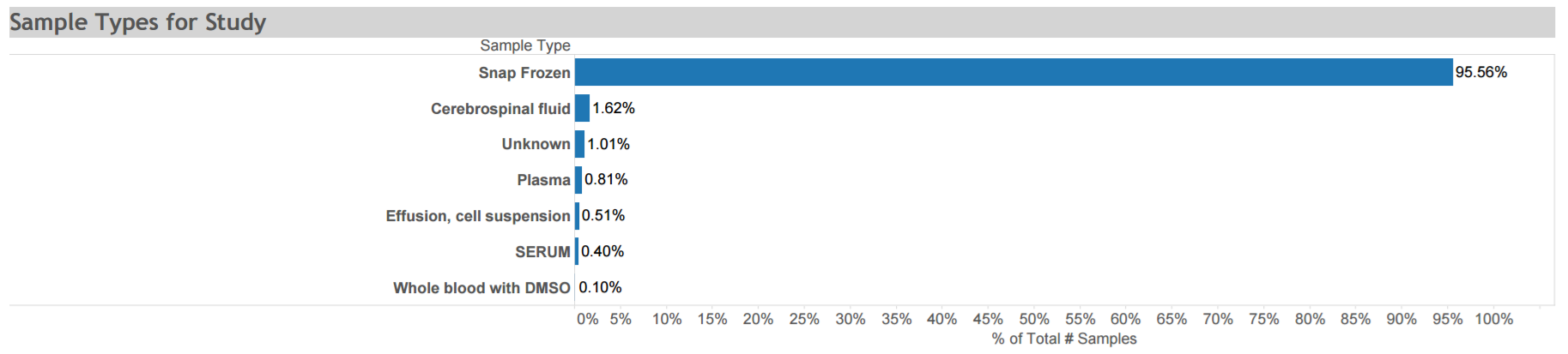
San Francisco Young Men’s Health Study: A population-based epidemiological risk factor study conducted in several ways among adult men who have sex with men in San Francisco. The cohort includes 581 men of varying status for HIV infection, KSHV infection, and KS. Data and biospecimens were collected at multiple time points from 1999 to 2002. Available specimens are depicted in the graphic. Click here for STUDY DETAIL
African
Samples and data from the African collections (through coordination with study PI, Dr. Jeff Martin) are available for late stage and confirmatory studies.
-
Antiretrovirals in Kaposi Sarcoma (ARKS)
-
Uganda AIDS Rural Treatment Outcomes (UARTO)
-
Epidemiology and Virology of KSHV in Zimbabwe
-
Transmission of KSHV to Children in South Africa
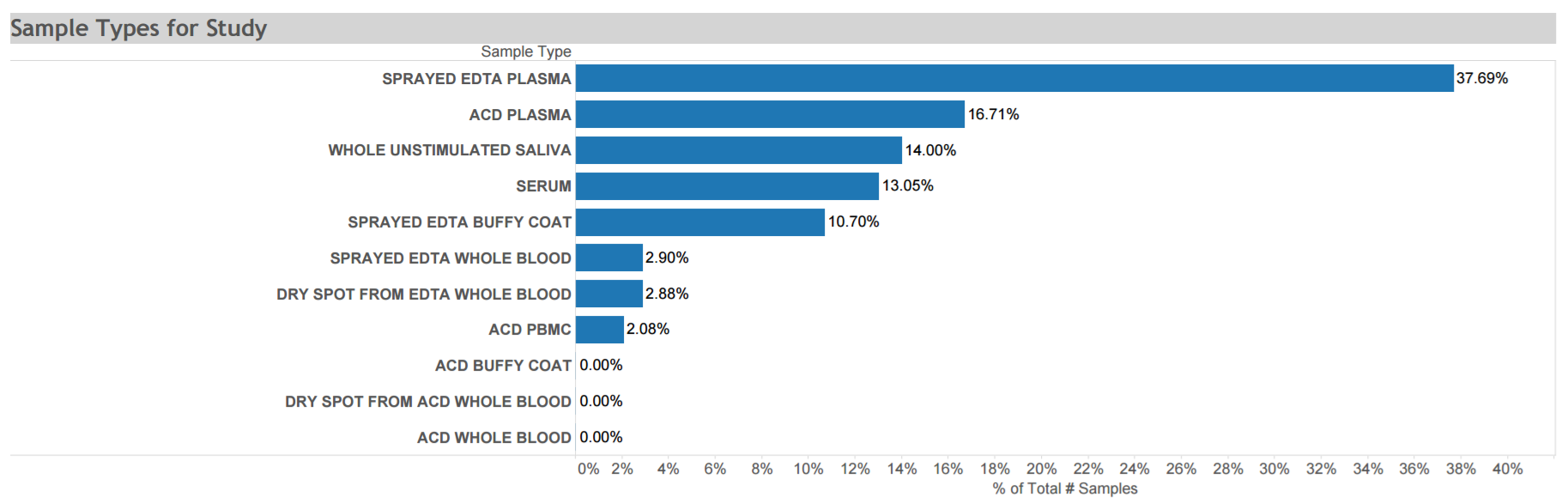
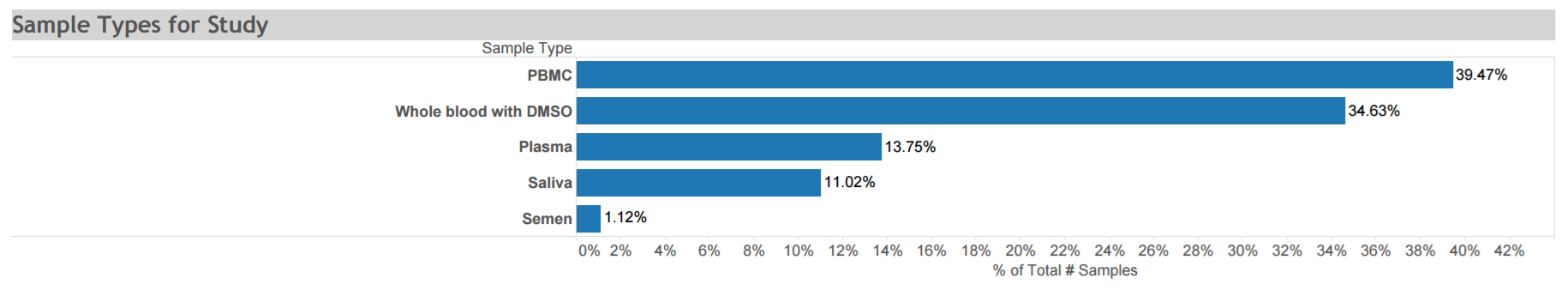
Antiretrovirals in Kaposi Sarcoma (ARKS): A randomized trial conducted from 2007 to 2011 to evaluate the efficacy of a protease inhibitor-based antiretroviral therapy regimen compared with a non-nucleoside reverse transcriptase inhibitor-based regimen to treat HIV-related histologically-confirmed KS in adults in Uganda. Data and biospecimens collected at multiple time points from this cohort of >200 participants are depicted in the graphic. Click here for STUDY DETAILS
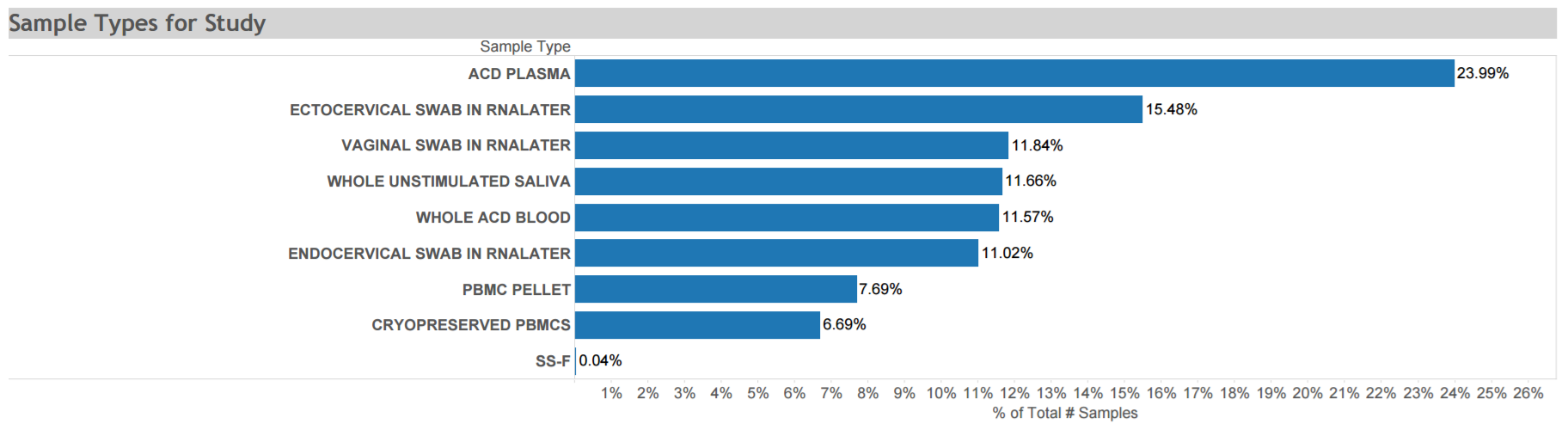
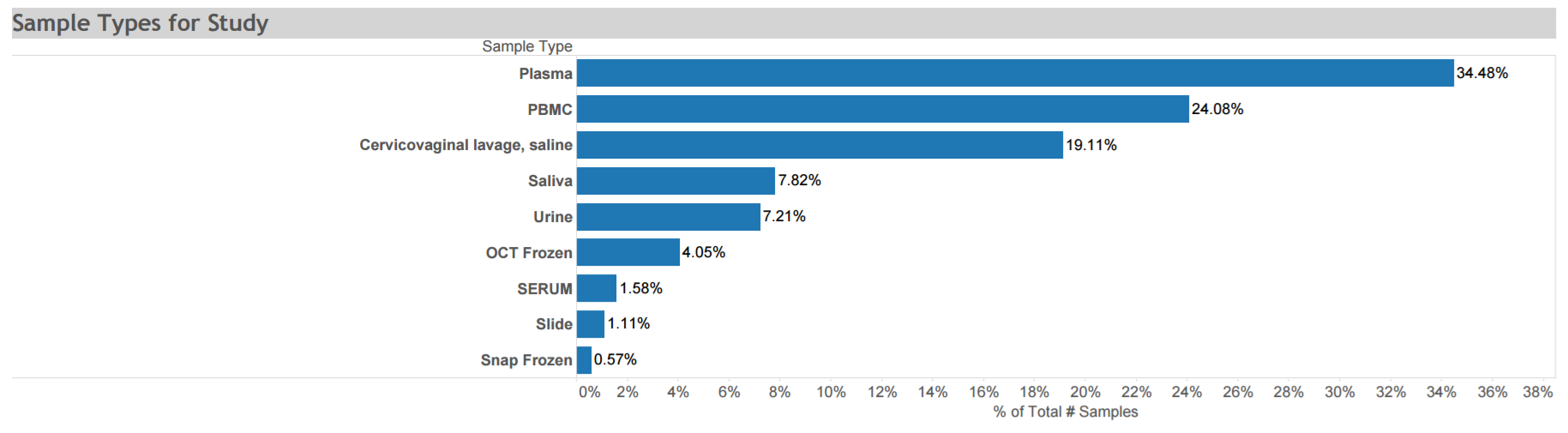
Uganda AIDS Rural Treatment Outcomes (UARTO): A prospective cohort study conducted from 2005 to 2010 to describe the virologic, immunologic, and clinical outcomes of antiretroviral therapy and factors associated with these outcomes among >700 adults recruited from a representative publicly funded municipal HIV/AIDS clinic in Mbarara, Uganda. Specimens collected at multiple time points are depicted in the graphic. Click here for STUDY DETAIL
Epidemiology and Virology of KSHV in Zimbabwe: A cross-sectional study conducted from 2001 to 2004 that evaluated the prevalence and determinants of KSHV infection and the pattern of KSHV shedding among >400 HIV+ and >200 HIV- adult women in Zimbabwe. KSHV antibody results and questionnaire data collected via direct interview are available and include demographic data, information about adult and childhood personal behaviors relevant to transmission as well as housing/residential factors, sexual history and pregnancies. Specimens collected at are depicted in the graphic. Click here for STUDY DETAIL
Transmission of KSHV to Children in South Africa: A cross-sectional study conducted in 2001 to 2004 that evaluated the prevalence and determinants of KSHV infection and shedding among >200 HIV+ and >700 HIV- children aged 1.5 to <9 years and their primary caregiver in rural and urban settings in Africa. KSHV antibody results are available. Questionnaire data collected via direct interview are consistent with the data collected in the Zimbabwe KSHV study in our inventory and include demographic data, information about adult and childhood personal behaviors relevant to transmission as well as housing/residential factors, sexual history and pregnancies. Available specimens are depicted in the graphic. Click here for STUDY DETAIL
Multisite Autopsy
The ACSR has a collection of multi-site frozen/FFPE autopsies as a novel source of tissues from 57 individuals who had been living with HIV and had been diagnosed with cancer or other conditions. Autopsy specimens are available from a spectrum of participants many within 6 hours of death, and most with multiple sites collected from cancer involved tissues as well as control uninvolved tissues.